Materials and equipment
The material and equipment characterization are reported in the supporting information.
Chemistry
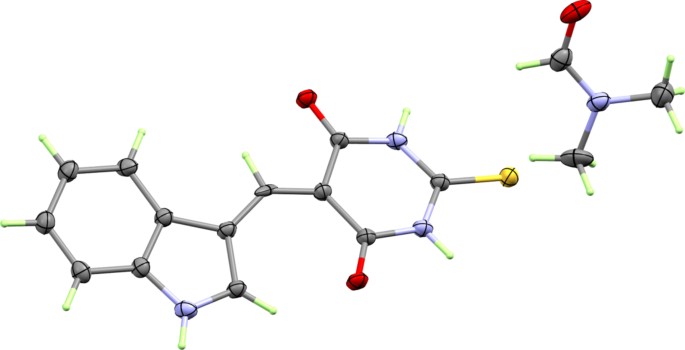
(5-((1H-indol-3-yl) methylene)−2-thioxodihydropyrimidine-4,6(1H,5H)-dione (3a)
A mixture of 2-thioxodihydro pyrimidine-4,6(1H,5H)-dione (1) (0.01 mol) and indole-3-carbaldehyde (0.01 mol) in 30 ml of absolute ethanol and drops of acetic acid was refluxed for 12 h, the excess solvent was separated under reduced pressure then the residue was poured into cold water (200 ml). The obtained solid was filtered off then re-dissolved in EtOH/DMF (3:1 v/v) mixture. This solution is left to evaporate at room temperature to give compound 3a as orange crystals (72% yield); Rf = 0.44 ( n-Hexane : ethyl acetate, 1:3, V/V); m.p = 279–280ºC; IR(KBr) νmax (cm − 1): 3437 (N-H) stretch, 1636(C = O), 1533(C = C), 1062(C = S) were observed as strong bands; 1 H NMR (500 MHz, DMSO-d6) δH: 12.90 (s, 1 H, NH), 12.22 (s, 1 H, NH), 12.16 (s, 1 H, NH), 9.54 (s, 1 H, CHO-DMF), 8.68 (s, 1 H, C = CH), 7.90 (s, 1 H, C = CH of indole), 7.84 (dd, J = 3.0 Hz, J = 6.0 Hz, 1 H, Ar-H), 7.56 (dd, J = 3.0 Hz, J = 6.0 Hz, 1 H, Ar-H), 7.29 (dd, J = 3.0 Hz, J = 5.0 Hz, 2 H, Ar-H), 2.83 (s, 3 H, CH3-DMF), 2.67 (s, 3 H, CH3-DMF); 13 C NMR (125 MHz, DMSO-d6) δC: 178.2 (C = S), 163.3, 162.8(C = O), 161.5, 145.0, 141.6, 137.1, 129.5, 124.5, 123.6, 118.3, 113.9, 112.9, 109.2 (Ar-C), 36.2, 31.2.
5-((1-benzyl-1H-indol-3-yl) methylene)−2-thioxodihydropyrimidine − 4,6(1H,5H)-dione (3b)
A mixture of 2-thioxodihydropyrimidine-4,6(1H,5H)-dione (1) (0.01 mol) and 1-benzyl-1H-indole-3-carbaldehyde (0.01 mol) in 30 ml of absolute ethanol and drops of acetic acid was refluxed for 12 h, the excess solvent was separated under reduced pressure then the residue was poured into cold water (200 ml). The obtained solid was filtered off and crystallized from ethanol and DMF to give compound 3b as orange powder (68% yield); Rf = 0.45 ( n-Hexane : ethyl acetate, 1:3, V/V); m.p = 309–311ºC; IR(KBr) νmax (cm − 1): 3437 (N-H) stretch, 1635(C = O), 1531(C = C), 1061(C = S) were observed as strong bands; 1 H NMR (500 MHz, DMSO-d6) δH: 12.21 (s, 2 H, NH), 9.67 (d, J = 4.5 Hz, 1 H, CHO-DMF), 8.65 (d, J = 4.5 Hz, 1 H C = CH), 7.91 (d, J = 4.0 Hz, 1 H, C = CH of indole), 7.88 (d, J = 4.5 Hz, 1 H, Ar-H), 7.67 (d, J = 5.5 Hz, 1 H, Ar-H), 7.22–7.33 (m, 8 H, Ar-H), 5.67 (d, J = 3.0 Hz, 2 H, –CH2Ph), 2.85 (d, J = 5.0 Hz, 3 H, CH3-DMF), 2.68 (d, J = 5.0 Hz, 3 H, CH3-DMF); 13 C NMR (125 MHz, DMSO-d6) δC: 178.3(C = S), 163.2, 162.8(C = O), 161.5, 144.3, 143.5, 137.2, 136.6, 130.3, 129.4, 128.6, 128.1, 124.7, 124.1, 118.7, 112.9, 112.2, 109.7 (Ar-C), 51.0(CH2Ph), 36.3, 31.3.
Preparation of Chitosan Nano-Formulation (CNPs)
Formulation of chitosan was prepared using ionic gelation method37,38. where half gram of chitosan was dissolved in 100 ml of 2% glacial acetic acid solution and was stirred for 2 h. Then the appropriate amount of synthesized hydrazones 3 was dissolved in 5 ml DMSO and stirred for 2 h. Following the preparation of a sodium tripolyphosphate anhydrous (TPP) solution (0.2% w/v in deionized water), the chitosan solution was vigorously stirred until equilibrium was reached, and then the thick chitosan emulsion was allowed to settle down and then subjected to centrifugation at 4,000 rpm for 30 min for CNPs 3 precipitation. For subsequent studies, the resulting precipitate was kept in sterile falcon tubes at 4 ºC.
Biological evaluation
Hemolytic activity assay (cytotoxicity on RBCs)
The hemolytic activity assay is reported in the supporting information.
Drugs
The oral administration of nitazoxanide (NTZ, Alinia, Romark labs, USA) at a dosage of 100 mg/kg per day began on day 10 following infection and continued for 14 days in a row39.
Oocysts Preparation
Cryptosporidium’s oocysts were acquired from the Parasitology Lab, Theodor Bilharz Research Institute, Giza, Egypt. Before infection, a hemocytometer was managed to concentrate and count the oocysts in PBS solution. Five mice were given 3000–3500 oocysts intraesophageally using a tuberculin syringe to sustain the organism cycle40,41.
Animals
This study was accomplished on 70Swiss albino male laboratory-bred mice (six-week-old, weighing approximately 20 gm). The mice were housed in regular circumstances after being acquired from Pharos University’s (PUA) animal home in Alexandria, Egypt. Before being infected with Cryptosporidium, the mice were given ten days to get used to the experimental setup. Mice were housed in plastic cages with clean wood-chip bedding and enough ventilation. Every mouse was kept in a regulated environment with a temperature of 21 ± 2 ◦C and a 12-hour light/dark cycle, mice grouping details was shown in in the supporting information.
Immunosuppression
The mice’s daily water intake was calculated to be around 4 milliliters, then the dexamethasone dose (0.25 mg/kg/day) was administered orally with drinking water (at a concentration of 62.5 µg/ml) for 14 succeeding days prior to infection with C. parvumoocysts. The immunosuppressed mice persisted in receiving the dexamethasone’s dose throughout the experiment42.
Infection
According to Rasmussen and Healey43, the oocyst suspension (104 oocysts/mL) was made in distilled water, who stated that a mouse infection requires 104 oocysts43.
Study design
Animals were distributed equally into Seven groups of 10 mice. At the ending of the experiment, animals were scarified to perform parasitological, ultrastructural and histopathological studies. Mice grouping details is reported in the supporting information.
Parasitological examination.
Fresh fecal pellets were gathered from each mouse group on day 24, the latest day of the experiment, and labeled with the number of oocysts. For each sample was suspended in ddH2O and then homogenized. Then, made a fecal smear of 1 mg feces which was stained by the amended Ziehl–Nelsen staining method. The stained fecal smear was scanned microscopically; and the Cryptosporidium oocysts were calculated, the average number of oocysts per milligram of feces for every mouse was recorded for every group.
Histopathological study.
The intestines of the sacrificed animals were dissected, cleaned with saline to remove any unwanted material, and then dry out on filter paper. Before being embedded in paraffin, the specimens were divided into pieces and preserved for 24 h in 5% paraformaldehyde. The samples were break into 4 μm-thick pieces and stained with hematoxylin-eosin utilizing the standard processing methodology of Bancroft and Gamble (2008). Histopathological attributes were seen using a light microscope (Lecia, DM750 P) with a camera-equipped section examination. Furthermore, quantitative assessment was evaluated utilizing intestinal morphometric analysis.
Ethical approval and the euthanasia process:
The present experiment was accomplished in accordance with the Animal Care and Use Committee at the Faculty of Pharmacy (unit of research ethics approval committee (UREAC)), Pharos University in Alexandria (PUA 04/404/26/09/202) and was in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The mice were given intraperitoneal injections of ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg) to result in mice anesthesia.








Leave a Reply